86
| WORLD FERTILIZER |
NOVEMBER 2016
scrubber system. A regulated amount of reagent will be mixed
in the re-circulating liquid to maintain a set-point pH level.
The contaminant in the gas will react with the reagent of
choice, forming ammonium salts. The reaction product will
be carried in the re-circulating liquid. Discharge of
concentrated usable ammonium salt is regulated by set-point
conductivity or density monitoring.
Process control with acid reagents
Nitric acids and sulfuric acids are most commonly used to
neutralise the ammonia gas after absorption into the scrubbing
liquid to form ammonium nitrate (NH
3
NO
3
) or ammonium
sulfate ((NH
4
)
2
SO
3
), respectively. The overall equation for each
reaction is shown in Equation 1 and Equation 2. The reagent is
added to maintain a pH set-point. Conductivity is continually
measured and is used to maintain a set-point concentration by
continually bleeding concentrated, usable ammonium salt
solution. Both (NH
4
)
2
SO
3
and NH
3
NO
3
are usable byproducts,
which reduce waste generation.
1. Ammonia removal with nitric acid:
NH
3(g)
+ HNO
3(aq)
→
NH
4
NO
3(aq)
2. Ammonia removal with sulfuric acid:
2 NH
3(g)
+ H
2
SO
4(aq)
→
(NH
4
)
2
SO
3 (aq)
Plant water or a dilute waste liquid stream can be used as
makeup water. This will provide for additional benefits by
converting a wastewater stream, which may need to be
evaporated, to a usable concentrated ammonium salt solution.
Process control with carbon dioxide
Using CO
2
to control ammonia provides a double benefit by
reducing CO
2
emissions into the atmosphere and generating a
usable byproduct. Carbon dioxide gas is bubbled through the
recirculating liquid (Figure 3) after ammonia absorption into
the scrubbing liquid to form ammonium bicarbonate. The
overall equation of this reaction is shown in Equation 3.
Since the CO
2
and NH
3
are in the gas phase, decreasing
the process temperature will increase the solubility and
therefore concentration, which is favourable for the reaction.
Furthermore, decreasing the temperature decreases the Gibbs
free energy and the reaction is thermodynamically more
favourable. In addition, the production of ammonium
bicarbonate is more thermodynamically favourable than
ammonium carbonate at all temperatures as governed by the
Gibbs free energy at standard conditions.
3. Ammonia removal with carbon dioxide:
2NH
3(g)
+ H
2
O + CO
2(g)
→
NH
4
HCO
3(aq)
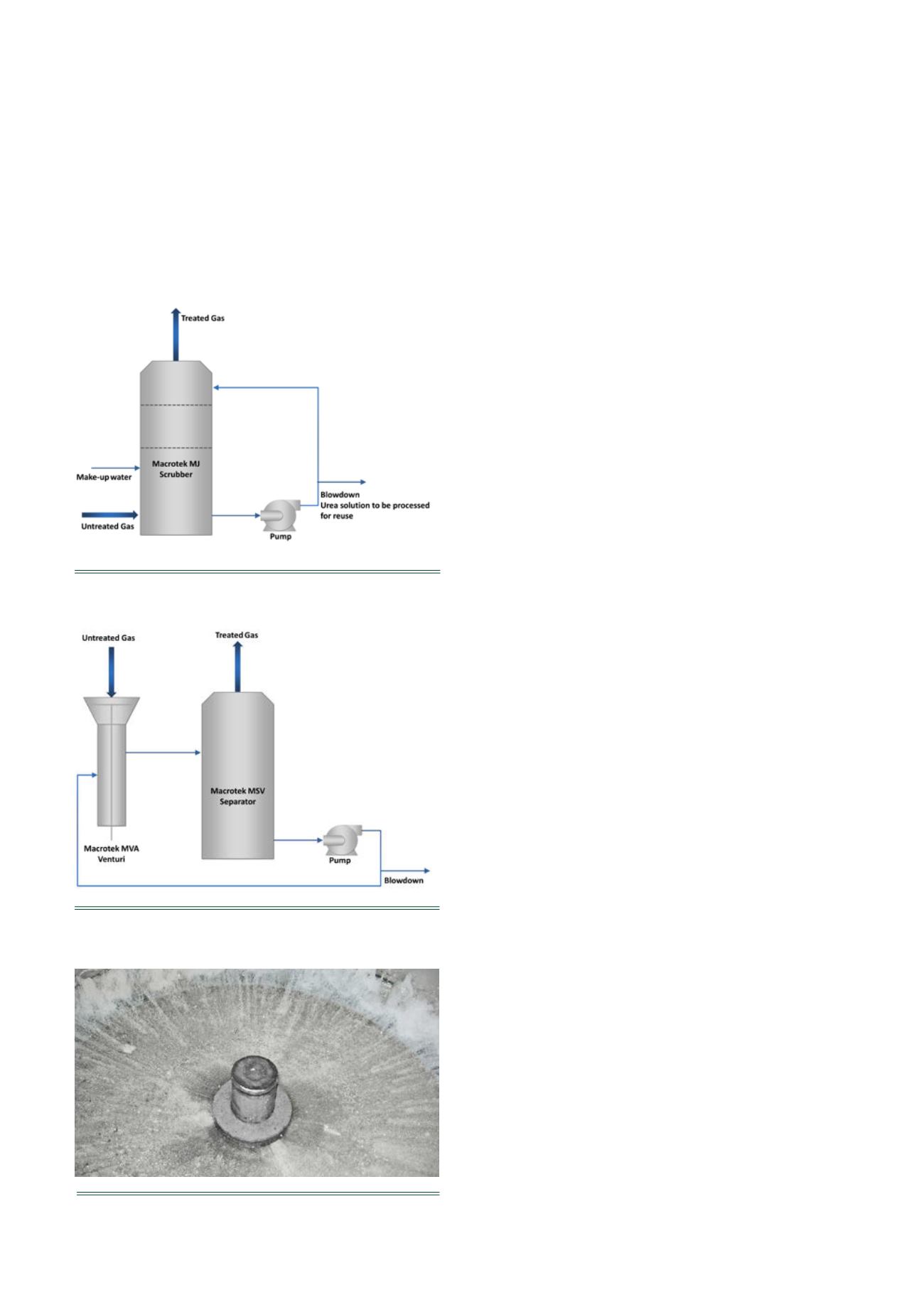
Plate-tray scrubber for urea collection
The Macrotek plate-tray scrubber (model MJ) has been
successfully applied to a diverse range of industrial
emissions (Figure 4). The system has no moving parts and
scrubber internals are readily accessible for routine
maintenance or replacement. High efficiencies for
particulate collection and mass transfer rates for gas
absorption are achieved at low pressure drops and low
liquid consumption rates. The scrubber uses the
impingement plate design, where gas to be scrubbed jets
upward through a flooded perforated plate colliding with a
wetted target directly above each orifice. The highly
turbulent action of these jets creates a uniform gas-liquid
contact zone for maximum urea capture.
Process control
As the urea in the untreated gas absorbs into the scrubbing
liquid and the urea concentration increases, a density
set-point is maintained by continually bleeding concentrated
urea solution back to the process for re-use.
Figure 4.
Process flow diagram of Macrotek MJ plate-tray
scrubber.
Figure 5.
Process flow diagram of Macrotek MVA venturi
scrubber.
Figure 6.
Flooded disc detail.


