84
| WORLD FERTILIZER |
NOVEMBER 2016
This article will describe several applications for the
treatment and control of ammonia and urea emissions,
including CO
2
injection and acid neutralisation. In all cases,
the objective is to combine and control emissions, and
generate a usable byproduct.
Ammonia and urea production and
emission sources
Ammonia (NH
3
) is produced by reacting the nitrogen in the air
with hydrogen from a hydrocarbon source, most commonly
natural gas. During the steam reforming process of natural gas,
carbon dioxide (CO
2
) is a generated waste byproduct, which is
often vented to the atmosphere.
Urea (NH
2
CONH
2
) is synthesised by reacting CO
2
and
liquid ammonia to produce ammonium carbamate, which is
dehydrated into urea. The urea is concentrated and sent to a
prill tower or granulator to produce solid urea.
Major ammonia emission sources originate from the
reaction process as well as from ammonia tank farms, while
major urea dust emission sources originate from the prill
tower or granulator.
Why remove ammonia and urea?
Ammonia is a colourless alkaline gas that has an odour
threshold between 5 – 50 ppm (OSHA). Not only is the odour
pungent, but it is also toxic if inhaled. Furthermore, ammonia
is corrosive to skin, eyes and the respiratory tract. Under
certain conditions, ammonia is flammable. It is very soluble in
water, forming ammonium hydroxide (NH
4
OH). Urea is a white
organic compound (particulate) and also very soluble in
water. Urea is also an irritant to skin, eyes and the respiratory
tract and is hazardous in case of ingestion or inhalation.
It is clear that the removal of ammonia gas and urea dust
from emission to the atmosphere is important for the
environment and human health. In addition, ammonia and
urea are also valuable products and it is therefore favourable
to optimise recovery for reuse.
Scrubber technologies and process control
Selection of the optimum control technology is dependent
on many factors, such as contaminant properties, required
removal efficiency, reagent, etc. In this section, three different
control technologies are summarised. For gases such as
ammonia, packed-bed scrubbers offer optimum mass transfer
due to the increased surface area provided by the packing
media. For readily soluble particulate, such as urea, plate-tray
scrubbers offer extended contact time of the liquid and gas
at each stage for dissolution of soluble salts into the
recirculating liquid, while venturi scrubbers achieve collection
of particulate by inertial impaction.
Packed bed for optimum mass transfer and
absorption of ammonia
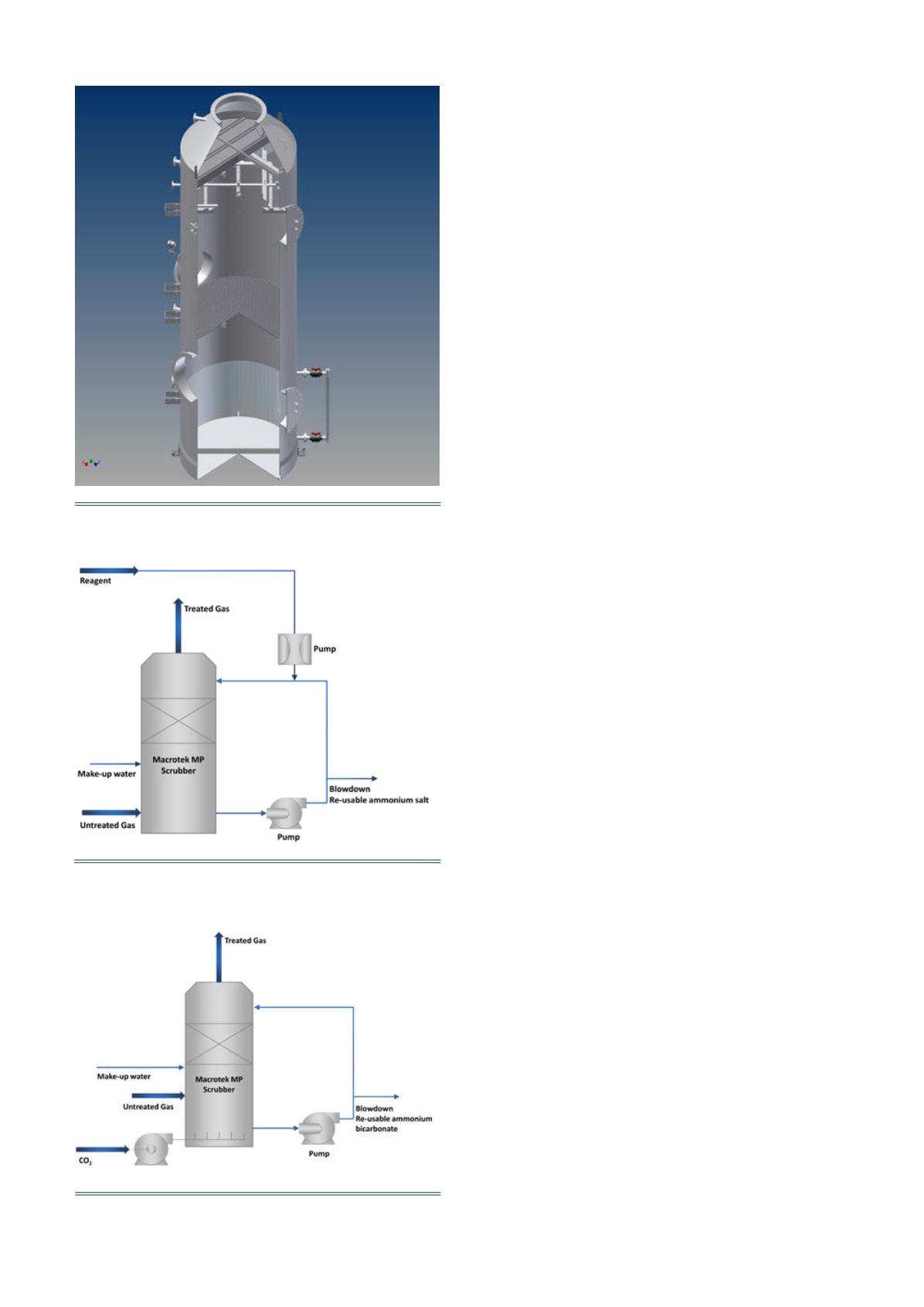
The Macrotek packed-bed scrubber (model MP in Figure 1)
uses a vertical counter-current design for the highly efficient
absorption of gases (Figure 2). In the vertical counter-current
design, gas flows upward while scrubbing liquid flows down
through a liquid distributor. The scrubber uses random
packing for enhanced mass transfer and absorption of gases.
A high-efficiency mist eliminator removes entrained liquids.
This is an essential step in the overall performance of the
Figure 3.
Removal of ammonia with carbon dioxide.
Figure 1.
Model of MP.
Figure 2.
Process flow diagram of Macrotek MP
packed-bed scrubber.


